The Documents Used to Develop Professional Research Guidelines Are Based on What Article?
Clifton Chow, Ph.D., Consultant - Anthony Petrosino, Ph.D., WestEd
The motion towards bear witness-based policy has focused on generating trustworthy evidence upon which to base decisions. For instance, a critical component of the evidence-based policy motion has been the support for more rigorous primary studies with strong "internal validity" such as randomized experiments and well-controlled quasi-experiments. Some other critical component has been the increased importance of transparent and explicit methods for synthesizing research such as systematic reviews and meta-analyses.
But once the scientific community has generated trustworthy research findings, how can this research exist used to generate policy and exercise guidance? Processes for doing this in a systematic, defensible and accessible mode has presented a challenge across different disciplines and fields. This briefing summarizes some of examples from different fields on the evolution of such processes.
The Importance of Guidelines and Minimizing Bias
The importance of guidelines development was articulated past the U.Southward. Centers for Disease Control:
Guidelines affect exercise, policy, and the allocation of resources on a wide scale. Thus, it is critical that recommendations included in guidelines documents are based on an objective cess of the best available show. Systematic literature reviews and rigorous methods used to rate the quality of bear witness can assistance in reducing scientific bias by increasing the probability that high‐quality, relevant evidence is considered. Even so, guideline evolution involves more than than assessing scientific bear witness. Developers also apply practiced stance to interpret inquiry and offering insights from practice. If not gathered carefully, practiced stance has the potential to bias the evidence synthesis and decision‐making process (come across Appendix A for farther elaboration by the CDC on selecting work group experts and the consensus approach).
Identifying Guidelines Evolution Documents
To apace place some different approaches, we solicited information from over 50 colleagues beyond fields of pedagogy, justice, public health, psychology, and folklore. Nosotros received fifteen documents and several electronic exchanges from these colleagues to identify how research evidence has been used to create public policy and exercise guidelines. These are common approaches, but no systematic search of the literature was undertaken to identify all relevant approaches. Of the 15 documents,i seven provided sufficient data to understand the framework proposed to move from research to policy recommendation. We begin with a word of six prominent guidelines creation documents.
6 Approaches to Guidelines Development
In this department, we summarize six approaches to creating guidelines: (1) The National Academies Study Process; (2) The Establish for Education Sciences Do Guide; (iii) Emerging Consensus on Rating Quality of Testify and Forcefulness of Recommendations (GRADE); (4) British Columbia Handbook on Developing Guidelines and Protocols; (five) International Standards for Clinical Policy Guidelines; and (6) The National Found for Health and Care Excellence (Overnice) Guidelines process.
Social Sciences
National Academies Study Process
National Academies reports are frequently viewed as beingness valuable and credible because of checks and balances that are applied at every step in the study process to protect the integrity of the reports and to maintain public confidence in them. Experts are selected to serve on a pro bono footing (travel expenses are reimbursed) on committees to produce study reports. The Academies provide independent advice; external sponsors have no control over the behave of a written report once the statement of task and budget are finalized. Study committees assemble information from many sources in public meetings but they carry out their deliberations in individual in society to avoid political, special involvement, and sponsor influence.
The process for producing a report written report involves the following:
Defining the written report. Academies' staff and members of their boards work with sponsors to make up one's mind the specific gear up of questions to be addressed by the study in a formal "statement of task," as well as the duration and cost of the study. The statement of task defines and bounds the scope of the study, and it serves as the basis for determining the expertise and the balance of perspectives needed on the committee.
Committee Pick and Approving. All committee members serve every bit individual experts, not as representatives of organizations or interest groups. Each fellow member is expected to contribute to the project on the footing of his or her own expertise and good judgment. A committee is non finally canonical until a thorough balance and conflict-of-interest give-and-take is held at the showtime meeting, and any problems raised in that discussion or by the public are investigated and addressed. Full details on how commission members are selected tin can be found in Appendix B.
Commission Meetings, Data Gathering, Deliberations, and Drafting the Written report. Study committees typically assemble data through: i) meetings that are open to the public and that are announced in advance through the Academies' website; 2) the submission of information past outside parties; 3) reviews of the scientific literature; and iv) the investigations of the committee members and staff. In all cases, efforts are made to solicit input from individuals who have been direct involved in, or who have special knowledge of, the problem under consideration.
Written report Review. As a final cheque on the quality and objectivity of the study, all Academies reports whether products of studies, summaries of workshop proceedings, or other documents must undergo a rigorous, independent external review past experts whose comments are provided anonymously to the commission members. The Academies recruit contained experts with a range of views and perspectives to review and annotate on the draft report prepared by the committee. The review process is structured to ensure that each report addresses its approved report charge and does not go beyond it, that the findings are supported by the scientific testify and arguments presented, that the exposition and system are effective, and that the report is impartial and objective.ii
Instruction
Plant for Education Sciences Practice Guides
A panel comprised of research experts, policymakers and practitioners is convened (generally around v persons). The console works with staff from the IES What Works Clearinghouse (WWC) to cull through the research bear witness. The WWC standards divide studies into those that come across minimum standards (either randomized controlled trials or quasi-experiments with strong evidence of group equivalence at baseline). All other studies are considered to not meet minimum standards. The studies are ranked according to these WWC bear witness standards. The panel meets several times (sometimes over several days) to weigh the evidence and construction recommendations. Each recommendation comes with a weak-to-strong qualifier that signals the extent to which conclusions were based on WWC-acceptable standards, or those that did not. When panels are not able to come to consensus, WWC management gets involved to make up one's mind resolutions and to push the process forward.
Health Care
Grading of Recommendations Assessment, Development and Evaluation (GRADE)
In 2000, a working group began as an breezy collaboration of people with an interest in addressing the shortcomings of grading evidence in health care. The working grouping developed an arroyo to grading quality of evidence and forcefulness of recommendations. Many international organizations take provided input into the evolution of the approach and have started using information technology (e.grand., the World Health Organization, the Cochrane Collaboration and more than 25 other organizations). GRADE was not adult for generating specific guidelines but is a process that was developed so that any organization can use it to create its own set of recommendations and standards. There are now literally dozens of articles on the GRADE procedure, and this briefing only discusses the overarching framework.
The commencement consideration in GRADE is a determination of whether the scientific evidence is of high quality (i.e., the bear witness indicates that the chances for desirable furnishings outweighs the chances for adverse effects). The 2nd consideration is to use this scientific evidence to produce a uncomplicated, transparent rating of the strength of the evidence supporting each recommendation (e.1000., "stiff" or "weak").
The GRADE approach tin be summarized as follows:
- The overall quality of show should be assessed for each important result and expressed using four (e.g. loftier, moderate, low, very depression) or, if justified, three (e.thousand. high, moderate, and very low and low combined into depression) categories. These are defined as follows:
- High quality— Farther research is very unlikely to change our confidence in the gauge of effect
- Moderate quality— Further inquiry is probable to accept an important bear upon on our conviction in the judge of upshot and may modify the estimate
- Low quality— Further research is very likely to have an of import impact on our confidence in the judge of result and is likely to alter the approximate.
- Very low quality— Any approximate of effect is very uncertain
- Evidence summaries (narrative or in table format) should exist used as the basis for judgments about the quality of evidence and the strength of recommendations. Ideally, these should be based on systematic reviews. At a minimum, the evidence that was assessed and the methods that were used to place and appraise that show should be clearly described.
- Explicit consideration should be given to each of the Course criteria for assessing the force of a recommendation (the balance of desirable and undesirable consequences, quality of testify, values and preferences, and resources use) and a general approach should be reported (east.g. if and how costs were considered, whose values and preferences were causeless, etc.).
- The strength of recommendations should be expressed using two categories (weak/conditional and potent) for or against a treatment option and the definitions for each category should be consistent with those used past GRADE. Unlike terminology to express weak/conditional and stiff recommendations may be used, although the interpretation and implications should be preserved. These decisions should be explicitly reported.
- Stiff: Based on the available bear witness, if clinicians are very sure that benefits do, or do not, outweigh risks and burdens they will brand a strong recommendation.
- Weak: Based on the available evidence, if clinicians believe that benefits and risks and burdens are finely counterbalanced, or appreciable uncertainty exists about the magnitude of benefits and risks, they must offering a weak recommendation. In addition, clinicians are becoming increasingly aware of the importance of patient values and preferences in clinical decision making. When, beyond the range of patient values, fully informed patients are liable to make different choices, guideline panels should offer weak recommendations.
British Columbia Handbook on Developing Guidelines and Protocols
The Canadian Guidelines and Protocols Advisory Committee (GPAC) is charged with developing clinical policy guidelines and recommendations for the British Columbia Province's Ministry building of Health. Their procedure in judging the state of evidence involves identifying the evidence from meta-analyses or other quantitative systematic reviews. If the systematic reviews on the topic are not yet available, the Committee conducts its own literature searches for private studies, preferably randomized controlled trials (RCTs). If this testify is too unavailable, recommendations are based on the "best available" bear witness.
One feature of GPAC is the way information technology assigns expert work groups. A separate workgroup is formed for a specific clinical guideline and is composed of general practitioners, relevant medical specialists and often a pharmacist from the ministry of health. The workgroup is overseen by a research officer (who may or may not exist the chair). The workgroup could be disbanded after an initial guideline has been developed and a new workgroup formed for subsequent revision. This process addresses the trouble of expert or "scientific bias" noted by many observers, the trend for guidelines to exist based overwhelmingly on the opinions of scientific experts to the exclusion of input by practitioners.
International Standards for Clinical Policy Guidelines
The American Higher of Physicians outlined some issues in developing clinical guidelines that included variations in quality, limitations of systematic reviews, and lack of transparency and acceptable documentation of methods. To accost these brusque-comings, the ACP created a set of recommendations for guideline creation and advocated for a utilize of a panel to form recommendations from research (i.east. the panel should include diverse and relevant stakeholders, such as health professionals, methodologists, experts on a topic, and patients). ACP also presented a set up of ruling principles for the creation of guidelines including:
1. A guideline should depict the process used to reach consensus among the console members and, if applicative, approval by the sponsoring organisation. This process should be established before the start of guideline development.
2. A guideline should clearly describe the methods used for the guideline development in detail.
3. Guideline developers should utilise systematic evidence review methods to identify and evaluate evidence related to the guideline topic.
4. A guideline recommendation should be clearly stated and based on scientific evidence of benefits; harms; and, if possible, costs.
5. A guideline should utilize a rating arrangement to communicate the quality and reliability of both the evidence and the forcefulness of its recommendations.
6. A guideline should include an expiration date and/or describe the process that the guideline groups will use to update recommendations.
7. A guideline should disclose fiscal support for the development of both the evidence review likewise equally the guideline recommendations
The National Institute for Health and Care Excellence (Nice)
The National Institute for Health and Intendance Excellence (Squeamish) in the UK makes bear witness-based recommendations on a wide range of topics, from preventing and managing specific conditions, improving health, managing medicines in different settings, to providing social care and support to adults and children, and planning broader services and interventions to ameliorate the health of English communities. The Prissy promotes both individualized intendance and integrated care (for example, by covering transitions between children'due south and adult services and betwixt health and social services).
Nice guidance is based on the best available evidence of what works and what it costs--and information technology is developed past Committees of experts. The NICE uses both scientific and other types of evidence from "multiple sources, extracted for different purposes and through different methods… within an ethical and theoretical framework." Evidence is classified into:
Scientific bear witness: which is defined as "explicit (codification and propositional), systemic (uses transparent and explicit methods for codifying), and replicable (using the same methods with the same samples will lead to the same results). Information technology tin be context-complimentary (applicable generally) or context-sensitive (driven past geography, fourth dimension and situation)".
Colloquial testify: substantially derived from expert testimony, stakeholder stance and necessarily value driven and subjective.
The evidence is then debated by a committee and the guidance developed and agreed upon. Ane feature of NICE is that clinical show is augmented by economic bear witness in forming judgments for guidelines. In that location are many documents on Overnice and an extensive manual. Appendix C provides a diagram of the NICE guideline creation procedure, and a summary of its cadre features.
Full general Learnings from the Half-dozen Approaches
Table 1 provides a summary of some characteristics of the six approaches described here. These characteristics include the field/discipline in which the guidelines were adult, whether a deliberating body was used to develop the guidelines, and whether the bear witness and the strength of the recommendation were rated.
| Approach | Field/Discipline | Type of Deliberating Body | Rating of Evidence? | Strength of Recommendation? |
| National Academies Study Reports | Sciences, More Broadly | Committees of Experts | No | No |
| Found for Education Sciences Practice Guides | Education | 5-person Console | Yeah | Yes |
| Form | Health Care | Organizations | Yes | Yes |
| British Columbia Handbook | Health Care | Work Group | Yes | Yes |
| American College of Physicians | Health Care | Panel | Yep | Yes |
| UK-National Found of Clinical | Health Care | Committees of Experts | Yeah | Yep |
These six approaches advise some overarching characteristics to be considered when developing guidelines:
- transparent and explicit process for developing guidelines from inquiry and other evidence is optimal.
- Because research and other types of show varies in terms of quality, a rating is needed. This does non mean that guidelines cannot be developed if a solid research base of operations does not exist, but the strength of the evidence supporting the guidelines should be made explicit.
- In several of these guidelines developments approaches, systematic reviews of existing evidence are prioritized equally "best testify" to consider.
- To ensure that the guidelines are relevant and believable to the practice customs, an good input process that includes not-researchers (practitioners and policymakers) should exist function of the guidelines development procedure.
- 3a. Several documents recommend that steps be taken to minimize potential conflicts of interest and biases of consensus or practiced panels.
Conclusion
This briefing summarized some examples from unlike disciplines and fields on the development of processes for generating trustworthy research findings into policy and practise guidelines. From electronic exchanges with colleagues and documents we obtained from them we outlined six approaches on how research has been used to create recommendations for public policy and exercise guidelines. All of these approaches balance on a transparent process at every stage, from the formation of deliberating bodies that are diverse in expertise to the discussion on the nature of the evidence and judgment identify on their internal validity. No report that are relevant to the topic are excluded, even if they were non from randomized-controlled studies. Care was also given to ensure that panel members are unbias, including rotating team members. The intendance taken and the flexibility of including a diversity of evidence ensure that policy and guidelines adult can be trusted and practical.
Appendix A. U.Southward. Centers for Disease Control Advice for Developing Guidelines
Selecting Work Grouping Experts
Scientific bias may enter into guideline development when of import scientific perspectives are not adequately represented. Guideline developers should select work grouping members in such a way that all relevant disciplines and perspectives are included and that members of both the science and practice perspectives are represented. Having a multidisciplinary work group tin help ensure the bear witness is reviewed and interpreted past individuals with varying values, preferences, and perspectives and that the resulting recommendations are balanced.
Using Consensus Evolution Methods
Scientific bias may also arise when the opinions of piece of work group experts are not adequately represented. The work group members may have differences in professional person condition or scientific knowledge. Some work group members boss discussions more than than others. Because of these differences and other social processes that emerge in group decision making, ensuring that data is shared and opinions are fairly represented can exist challenging. Consensus development methods can help ensure that all expert perspectives are shared and that bias is counterbalanced. Consensus methods that might be considered include the Delphi method, the Nominal Group procedure, and the Glaser approach. These methods construction group interaction in means that bring consensus on recommendation statements; for example, by using an iterative process to solicit views through questionnaires, note cards, or written documents, reverberate views back to work group members systematically, and formulate final written recommendations. Regardless of the method used, systematic ways of gathering expert opinion, views, and preferences for recommendations can help to reduce bias.
Appendix B. National Academies Commission Choice Criteria
- An advisable range of expertise for the task. The committee must include experts with the specific expertise and feel needed to address the report's statement of task. One of the strengths of the Academies is the tradition of bringing together recognized experts from diverse disciplines and backgrounds who might non otherwise interact. These diverse groups are encouraged to conceive new means of thinking about a problem.
- A residuum of perspectives. Having the correct expertise is not sufficient for success. Information technology is also essential to evaluate the overall composition of the committee in terms of different experiences and perspectives. The goal is to ensure that the relevant points of view are, in the Academies' judgment, reasonably balanced so that the committee can deport out its charge considerately and credibly.
- Screened for conflicts of interest. All conditional committee members are screened in writing and in a confidential grouping word about possible conflicts of interest. For this purpose, a "conflict of interest" means whatsoever financial or other interest which conflicts with the service of the individual because it could significantly impair the individual'southward objectivity or could create an unfair competitive advantage for any person or organization. The term "disharmonize of interest" ways something more than than individual bias. There must be an involvement, ordinarily financial, that could be directly afflicted by the work of the committee. Except for those rare situations in which the Academies make up one's mind that a disharmonize of interest is unavoidable and promptly and publicly disembalm the conflict of interest, no private tin be appointed to serve (or go along to serve) on a commission of the establishment used in the development of reports if the individual has a conflict of involvement that is relevant to the functions to be performed.
- Point of View is unlike from Conflict of Interest. A point of view or bias is non necessarily a conflict of interest. Committee members are expected to have points of view, and the Academies attempt to residue these points of view in a mode deemed appropriate for the task. Commission members are asked to consider respectfully the viewpoints of other members, to reflect their ain views rather than be a representative of whatever arrangement, and to base their scientific findings and conclusions on the evidence. Each committee fellow member has the right to event a dissenting opinion to the report if he or she disagrees with the consensus of the other members.
- Other considerations. Membership in the NAS, NAE, or NAM and previous involvement in Academies studies are taken into account in commission selection. The inclusion of women, minorities, and young professionals are additional considerations.
Specific steps in the commission choice and approval process are as follows:
- Staff solicit an extensive number of suggestions for potential commission members from a broad range of sources, so recommend a slate of nominees.
- Nominees are reviewed and approved at several levels within the Academies; a provisional slate is then approved by the president of the National Academy of Sciences, who is likewise the chair of the National Research Quango.
- The conditional committee list is posted for public comment in the Current Projects System on the Spider web.
- The provisional committee members complete background information and disharmonize-of-involvement disclosure forms.
- The committee balance and conflict-of-interest discussion is held at the first committee coming together.
- Any conflicts of interest or issues of committee balance and expertise are investigated; changes to the committee are proposed and finalized.
- Committee is formally approved.
- Committee members continue to be screened for disharmonize of interest throughout the life of the committee.
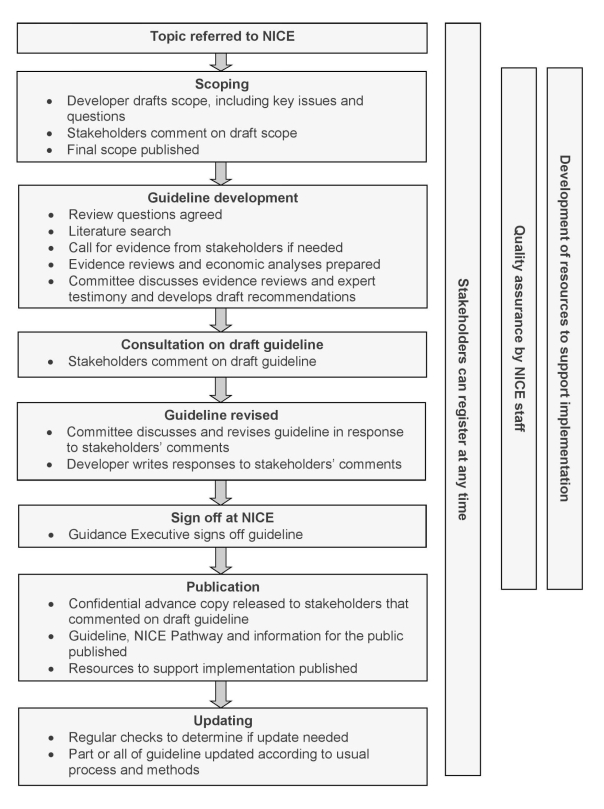
Relevant features of Nice
I. Commission Membership
In terms of participation in committees, Dainty also differs from other panels in that it includes lay members and public at-big. Lay members are defined equally those with personal experience of using health or care services, or from a community afflicted by an established or presently to exist considered guideline. In developing the guidelines, the Commission is the independent informational group that considers the evidence and develops the recommendations, taking into account the views of stakeholders. It may be a continuing Committee working on many guideline topics, or a topic-specific Committee put together to work on a specific guideline. NICE also advocates flexibility in calling for participation in the Committee. If needed for a topic, the Committee tin co-opt members with specific expertise to contribute to developing some of the recommendations. For example, members with feel of integrating delivery of services across service areas may too be recruited, particularly where the development of a guideline requires more flexibility than "conventional organisational boundaries" permit. If the guideline contains recommendations about services, NICE could call upon individuals with a commissioning or provider background in add-on to members from practitioner networks or local authorities.
Ii. Evidence
The Prissy approach towards evaluating clinical evidence differs from other approaches. In addition to clinical evidence, the committee is implored to besides take into business relationship other factors, such as the need to prevent discrimination and to promote equity. Similarly, Dainty recognizes that not all clinical research could or should issue in implementation; therefore, Nice has added an indication as to whether a procedure should but exist tested in further enquiry or that information technology be put forward for implementation. Factors that might prevent research from beingness implemented in do would be show that the committee considers to exist insufficient at the current fourth dimension. A 'research simply' recommendation is made if the prove shows that there are important uncertainties which may be resolved with additional evidence (presumably from clinical trials or real world settings).Evidence may as well indicates the intervention is unsafe and/or not efficacious, and the commission volition brand a recommendation, under those weather, non to use the process.
Iii. Economic Bear witness
An important feature in the NICE framework is its utilize of economic prove in guidelines development. There are ii primary considerations in drawing conclusions from economic studies for a given intervention. The first is that the methodology is sufficiently strong to avert the possibility of double-counting costs or benefits. NICE recommends that the way consequences are implicitly weighted should exist recorded openly, transparently and as accurately as possible. Cost–consequences analysis so requires the determination-maker to decide which interventions stand for the best value using a systematic and transparent process. A related process is that an incremental cost-effectiveness ratio (ICER) threshold be used whenever possible and that interventions with an estimated negative net present value (NPV) should non be recommended unless social values outweigh costs.
The second consideration Prissy put forward on using economic evidence in translating inquiry to clinical practice/policy concerns cost-minimization procedures. The commission took care to avoid blindly choosing interventions with the lowest costs by declaring that cost minimization can be used merely when the departure in benefits between an intervention and its comparator is known to exist small and the price difference is big. Given the criteria, Dainty believes that cost-minimisation analysis is only applicable in a relatively small-scale number of cases.
In sum, economic evidence estimating the value of the intervention should exist considered alongside clinical show, but judgment past social values (policy) should also be taken into account to avert choosing intervention merely because information technology is offered at the lowest cost.
IV. Producing Guidelines from Evidence
The terminal step in translating research evidence into practice and policy guidelines is drafting recommendations. Because many people read only the recommendations, the wording must be concise, unambiguous and easy to translate into practice by the intended audience. As a general dominion, the commission recommends that each recommendation or bullet point within a recommendation should contain only one primary activeness and be attainable as much every bit possible to a broad audience.
An of import guideline explicitly stated by Prissy is to indicate levels of uncertainty in the evidence. It is the only institution to have created a "Research recommendations process and methods guide," which details the approach to exist used to identify primal uncertainties and associated research recommendations. In considering which enquiry intervention or bear witness to put forrard for recommendation, the committee established guidelines that includes three levels of certainty:
1. Recommendations for activities or interventions that should (or should not) exist used
2. Recommendations for activities or interventions that could be used
3. Recommendations for activities or interventions that must (or must not) be used.
Bibliography
- CDC (2012). Reducing Scientific Bias from Expert Opinion in Guidelines and Recommendations Development.
- Class Working Group (2015). From Evidence to Recommendations: Transparent and Sensible. Retrieved from: http://www.gradeworkinggroup.org/.
- Guyatt, M.H. (2008). Grade: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336.
- Ministry building of Health of British Columbia (2014). Guidelines and Protocols Advisory Committee Handbook: Developing Clinical Practice Guidelines and Protocols for British Columbia.
- National Academies of Sciences, Engineering & Medicine (2015). Our Study Process: Ensuring Independent, Objective Advice. Washington, D.C. Retrieved from: http://www.nationalacademies.org/studyprocess/.
- Quaseem, A. Towards International Standards for Clinical Practice Guidelines. Presentation delivered past the Chair of the Guidelines for International Network, Scottish Charity.
- UK National Plant for Health and Care Excellence (2015). The Manual on Developing NICE Guidelines.
- Institute of Education Sciences (2015). What Works Clearinghouse. Retrieved from: http://ies.ed.gov/ncee/wwc/Publications_Reviews.aspx?f=All%20Publication%20and%20Product%20Types,iii.
Clifton Chow, Ph.D., Consultant - Anthony Petrosino, Ph.D., WestEd
The move towards evidence-based policy has focused on generating trustworthy evidence upon which to base decisions. For instance, a critical component of the show-based policy movement has been the support for more rigorous main studies with strong "internal validity" such every bit randomized experiments and well-controlled quasi-experiments. Another critical component has been the increased importance of transparent and explicit methods for synthesizing inquiry such as systematic reviews and meta-analyses.
Simply once the scientific community has generated trustworthy inquiry findings, how tin this enquiry be used to generate policy and practice guidance? Processes for doing this in a systematic, defensible and accessible way has presented a claiming across different disciplines and fields. This conference summarizes some of examples from dissimilar fields on the development of such processes.
The Importance of Guidelines and Minimizing Bias
The importance of guidelines development was articulated by the U.S. Centers for Affliction Command:
Guidelines affect practise, policy, and the allotment of resources on a wide scale. Thus, it is disquisitional that recommendations included in guidelines documents are based on an objective cess of the all-time bachelor evidence. Systematic literature reviews and rigorous methods used to rate the quality of show can help in reducing scientific bias past increasing the probability that high‐quality, relevant bear witness is considered. However, guideline evolution involves more than than assessing scientific evidence. Developers also employ expert opinion to interpret research and offering insights from practise. If not gathered carefully, expert opinion has the potential to bias the evidence synthesis and decision‐making process (see Appendix A for further elaboration by the CDC on selecting work group experts and the consensus arroyo).
Identifying Guidelines Evolution Documents
To rapidly identify some unlike approaches, nosotros solicited information from over 50 colleagues across fields of educational activity, justice, public wellness, psychology, and sociology. Nosotros received 15 documents and several electronic exchanges from these colleagues to place how research show has been used to create public policy and exercise guidelines. These are common approaches, but no systematic search of the literature was undertaken to identify all relevant approaches. Of the xv documents,i seven provided sufficient data to understand the framework proposed to move from enquiry to policy recommendation. Nosotros begin with a discussion of six prominent guidelines creation documents.
Half-dozen Approaches to Guidelines Development
In this section, we summarize six approaches to creating guidelines: (1) The National Academies Study Process; (2) The Institute for Education Sciences Practice Guide; (3) Emerging Consensus on Rating Quality of Prove and Force of Recommendations (GRADE); (4) British Columbia Handbook on Developing Guidelines and Protocols; (5) International Standards for Clinical Policy Guidelines; and (vi) The National Institute for Health and Care Excellence (NICE) Guidelines process.
Social Sciences
National Academies Report Procedure
National Academies reports are often viewed equally being valuable and credible because of checks and balances that are applied at every pace in the study process to protect the integrity of the reports and to maintain public confidence in them. Experts are selected to serve on a pro bono footing (travel expenses are reimbursed) on committees to produce written report reports. The Academies provide independent communication; external sponsors have no control over the conduct of a study once the argument of task and upkeep are finalized. Study committees assemble information from many sources in public meetings but they carry out their deliberations in individual in order to avert political, special interest, and sponsor influence.
The process for producing a study report involves the following:
Defining the study. Academies' staff and members of their boards work with sponsors to determine the specific set up of questions to be addressed by the study in a formal "statement of task," as well as the duration and cost of the report. The statement of chore defines and bounds the scope of the study, and information technology serves as the basis for determining the expertise and the balance of perspectives needed on the committee.
Committee Selection and Approving. All committee members serve as individual experts, non as representatives of organizations or involvement groups. Each member is expected to contribute to the project on the ground of his or her own expertise and expert judgment. A committee is not finally approved until a thorough residuum and conflict-of-involvement discussion is held at the get-go meeting, and any issues raised in that discussion or past the public are investigated and addressed. Full details on how committee members are selected can be found in Appendix B.
Committee Meetings, Information Gathering, Deliberations, and Drafting the Report. Report committees typically gather information through: one) meetings that are open to the public and that are appear in advance through the Academies' website; 2) the submission of information by outside parties; 3) reviews of the scientific literature; and 4) the investigations of the committee members and staff. In all cases, efforts are fabricated to solicit input from individuals who have been directly involved in, or who have special cognition of, the problem under consideration.
Report Review. Every bit a final check on the quality and objectivity of the study, all Academies reports whether products of studies, summaries of workshop proceedings, or other documents must undergo a rigorous, independent external review past experts whose comments are provided anonymously to the committee members. The Academies recruit independent experts with a range of views and perspectives to review and comment on the typhoon study prepared by the committee. The review process is structured to ensure that each written report addresses its approved written report charge and does non go across it, that the findings are supported by the scientific evidence and arguments presented, that the exposition and organisation are effective, and that the report is impartial and objective.ii
Education
Institute for Didactics Sciences Practise Guides
A panel comprised of research experts, policymakers and practitioners is convened (generally around 5 persons). The panel works with staff from the IES What Works Clearinghouse (WWC) to choose through the research bear witness. The WWC standards dissever studies into those that meet minimum standards (either randomized controlled trials or quasi-experiments with strong bear witness of grouping equivalence at baseline). All other studies are considered to not come across minimum standards. The studies are ranked according to these WWC testify standards. The panel meets several times (sometimes over several days) to weigh the bear witness and structure recommendations. Each recommendation comes with a weak-to-stiff qualifier that signals the extent to which conclusions were based on WWC-adequate standards, or those that did not. When panels are not able to come up to consensus, WWC management gets involved to decide resolutions and to push the process forward.
Health Care
Grading of Recommendations Cess, Development and Evaluation (GRADE)
In 2000, a working group began as an breezy collaboration of people with an interest in addressing the shortcomings of grading evidence in health intendance. The working grouping developed an arroyo to grading quality of evidence and strength of recommendations. Many international organizations take provided input into the development of the approach and have started using information technology (eastward.yard., the Globe Health Organization, the Cochrane Collaboration and more than 25 other organizations). Form was not developed for generating specific guidelines but is a process that was developed so that whatever organization tin use it to create its own set of recommendations and standards. There are at present literally dozens of articles on the Class process, and this conference only discusses the overarching framework.
The first consideration in Grade is a determination of whether the scientific bear witness is of high quality (i.e., the bear witness indicates that the chances for desirable furnishings outweighs the chances for adverse effects). The second consideration is to apply this scientific evidence to produce a elementary, transparent rating of the strength of the testify supporting each recommendation (e.g., "stiff" or "weak").
The Form approach can be summarized as follows:
- The overall quality of evidence should be assessed for each important outcome and expressed using four (e.g. loftier, moderate, low, very depression) or, if justified, iii (east.g. high, moderate, and very low and low combined into low) categories. These are divers as follows:
- Loftier quality— Further inquiry is very unlikely to change our conviction in the estimate of effect
- Moderate quality— Further research is probable to have an important impact on our conviction in the approximate of upshot and may change the estimate
- Low quality— Further inquiry is very probable to have an of import impact on our confidence in the gauge of result and is likely to change the estimate.
- Very low quality— Any estimate of effect is very uncertain
- Show summaries (narrative or in table format) should be used as the footing for judgments most the quality of prove and the strength of recommendations. Ideally, these should be based on systematic reviews. At a minimum, the prove that was assessed and the methods that were used to identify and appraise that evidence should be conspicuously described.
- Explicit consideration should be given to each of the Class criteria for assessing the strength of a recommendation (the balance of desirable and undesirable consequences, quality of testify, values and preferences, and resources apply) and a full general arroyo should be reported (eastward.thou. if and how costs were considered, whose values and preferences were causeless, etc.).
- The strength of recommendations should be expressed using 2 categories (weak/provisional and strong) for or confronting a treatment pick and the definitions for each category should exist consistent with those used past Class. Different terminology to express weak/conditional and stiff recommendations may be used, although the interpretation and implications should be preserved. These decisions should be explicitly reported.
- Strong: Based on the available testify, if clinicians are very certain that benefits exercise, or exercise non, outweigh risks and burdens they will brand a strong recommendation.
- Weak: Based on the bachelor evidence, if clinicians believe that benefits and risks and burdens are finely counterbalanced, or observable doubtfulness exists about the magnitude of benefits and risks, they must offer a weak recommendation. In addition, clinicians are becoming increasingly aware of the importance of patient values and preferences in clinical decision making. When, beyond the range of patient values, fully informed patients are liable to make different choices, guideline panels should offer weak recommendations.
British Columbia Handbook on Developing Guidelines and Protocols
The Canadian Guidelines and Protocols Advisory Commission (GPAC) is charged with developing clinical policy guidelines and recommendations for the British Columbia Province's Ministry of Health. Their procedure in judging the land of show involves identifying the evidence from meta-analyses or other quantitative systematic reviews. If the systematic reviews on the topic are not yet bachelor, the Committee conducts its own literature searches for individual studies, preferably randomized controlled trials (RCTs). If this evidence is too unavailable, recommendations are based on the "best bachelor" evidence.
One feature of GPAC is the way it assigns skillful work groups. A carve up workgroup is formed for a specific clinical guideline and is composed of general practitioners, relevant medical specialists and frequently a pharmacist from the ministry of health. The workgroup is overseen by a research officer (who may or may not exist the chair). The workgroup could be disbanded later on an initial guideline has been adult and a new workgroup formed for subsequent revision. This procedure addresses the problem of skillful or "scientific bias" noted by many observers, the tendency for guidelines to be based overwhelmingly on the opinions of scientific experts to the exclusion of input past practitioners.
International Standards for Clinical Policy Guidelines
The American College of Physicians outlined some bug in developing clinical guidelines that included variations in quality, limitations of systematic reviews, and lack of transparency and adequate documentation of methods. To address these short-comings, the ACP created a set of recommendations for guideline creation and advocated for a use of a panel to class recommendations from research (i.due east. the panel should include diverse and relevant stakeholders, such as wellness professionals, methodologists, experts on a topic, and patients). ACP also presented a set of ruling principles for the creation of guidelines including:
i. A guideline should describe the procedure used to reach consensus amid the panel members and, if applicative, approval by the sponsoring organization. This procedure should be established before the start of guideline development.
2. A guideline should clearly describe the methods used for the guideline development in detail.
three. Guideline developers should use systematic evidence review methods to identify and evaluate evidence related to the guideline topic.
4. A guideline recommendation should be clearly stated and based on scientific bear witness of benefits; harms; and, if possible, costs.
5. A guideline should use a rating organization to communicate the quality and reliability of both the evidence and the force of its recommendations.
6. A guideline should include an expiration date and/or describe the process that the guideline groups will apply to update recommendations.
7. A guideline should disclose financial back up for the development of both the evidence review as well equally the guideline recommendations
The National Constitute for Wellness and Intendance Excellence (Dainty)
The National Institute for Health and Intendance Excellence (NICE) in the UK makes evidence-based recommendations on a wide range of topics, from preventing and managing specific conditions, improving health, managing medicines in different settings, to providing social care and support to adults and children, and planning broader services and interventions to improve the health of English language communities. The Dainty promotes both individualized care and integrated care (for example, by covering transitions between children'due south and adult services and between health and social services).
NICE guidance is based on the all-time available evidence of what works and what it costs--and it is developed by Committees of experts. The Prissy uses both scientific and other types of evidence from "multiple sources, extracted for dissimilar purposes and through unlike methods… within an upstanding and theoretical framework." Bear witness is classified into:
Scientific evidence: which is defined as "explicit (codification and propositional), systemic (uses transparent and explicit methods for codifying), and replicable (using the same methods with the same samples will lead to the aforementioned results). It tin be context-free (applicable more often than not) or context-sensitive (driven by geography, time and situation)".
Colloquial evidence: essentially derived from expert testimony, stakeholder opinion and necessarily value driven and subjective.
The evidence is and then debated by a committee and the guidance developed and agreed upon. One characteristic of NICE is that clinical testify is augmented by economic evidence in forming judgments for guidelines. There are many documents on Nice and an extensive transmission. Appendix C provides a diagram of the Dainty guideline cosmos procedure, and a summary of its cadre features.
Full general Learnings from the Six Approaches
Table 1 provides a summary of some characteristics of the six approaches described hither. These characteristics include the field/discipline in which the guidelines were developed, whether a deliberating trunk was used to develop the guidelines, and whether the evidence and the forcefulness of the recommendation were rated.
| Approach | Field/Bailiwick | Type of Deliberating Body | Rating of Testify? | Strength of Recommendation? |
| National Academies Written report Reports | Sciences, More Broadly | Committees of Experts | No | No |
| Institute for Educational activity Sciences Practise Guides | Education | 5-person Panel | Yeah | Yes |
| GRADE | Wellness Care | Organizations | Yes | Yes |
| British Columbia Handbook | Health Care | Piece of work Group | Aye | Yes |
| American Higher of Physicians | Health Care | Console | Yeah | Yes |
| UK-National Constitute of Clinical | Wellness Intendance | Committees of Experts | Yes | Yes |
These six approaches suggest some overarching characteristics to exist considered when developing guidelines:
- transparent and explicit process for developing guidelines from inquiry and other evidence is optimal.
- Because research and other types of bear witness varies in terms of quality, a rating is needed. This does not mean that guidelines cannot be developed if a solid enquiry base of operations does non exist, but the force of the testify supporting the guidelines should be fabricated explicit.
- In several of these guidelines developments approaches, systematic reviews of existing testify are prioritized as "best evidence" to consider.
- To ensure that the guidelines are relevant and conceivable to the practice community, an skillful input process that includes non-researchers (practitioners and policymakers) should be part of the guidelines development process.
- 3a. Several documents recommend that steps be taken to minimize potential conflicts of interest and biases of consensus or proficient panels.
Conclusion
This briefing summarized some examples from different disciplines and fields on the evolution of processes for generating trustworthy enquiry findings into policy and practise guidelines. From electronic exchanges with colleagues and documents we obtained from them we outlined six approaches on how research has been used to create recommendations for public policy and practice guidelines. All of these approaches residual on a transparent process at every stage, from the formation of deliberating bodies that are diverse in expertise to the give-and-take on the nature of the evidence and judgment identify on their internal validity. No study that are relevant to the topic are excluded, even if they were not from randomized-controlled studies. Intendance was likewise given to ensure that console members are unbias, including rotating team members. The intendance taken and the flexibility of including a variety of evidence ensure that policy and guidelines developed tin be trusted and applied.
Appendix A. U.Due south. Centers for Disease Command Advice for Developing Guidelines
Selecting Work Grouping Experts
Scientific bias may enter into guideline development when of import scientific perspectives are not fairly represented. Guideline developers should select work group members in such a manner that all relevant disciplines and perspectives are included and that members of both the science and practice perspectives are represented. Having a multidisciplinary work grouping can help ensure the testify is reviewed and interpreted by individuals with varying values, preferences, and perspectives and that the resulting recommendations are balanced.
Using Consensus Evolution Methods
Scientific bias may besides arise when the opinions of work group experts are not adequately represented. The work group members may have differences in professional condition or scientific cognition. Some piece of work grouping members dominate discussions more than others. Considering of these differences and other social processes that sally in group decision making, ensuring that information is shared and opinions are adequately represented can exist challenging. Consensus development methods tin can help ensure that all expert perspectives are shared and that bias is counterbalanced. Consensus methods that might be considered include the Delphi method, the Nominal Group process, and the Glaser approach. These methods structure group interaction in ways that bring consensus on recommendation statements; for example, past using an iterative process to solicit views through questionnaires, note cards, or written documents, reflect views dorsum to piece of work group members systematically, and formulate final written recommendations. Regardless of the method used, systematic ways of gathering expert opinion, views, and preferences for recommendations can help to reduce bias.
Appendix B. National Academies Committee Choice Criteria
- An appropriate range of expertise for the task. The committee must include experts with the specific expertise and feel needed to accost the study's statement of task. One of the strengths of the Academies is the tradition of bringing together recognized experts from diverse disciplines and backgrounds who might non otherwise interact. These various groups are encouraged to excogitate new ways of thinking about a problem.
- A balance of perspectives. Having the right expertise is not sufficient for success. It is likewise essential to evaluate the overall composition of the committee in terms of dissimilar experiences and perspectives. The goal is to ensure that the relevant points of view are, in the Academies' judgment, reasonably balanced and so that the committee can conduct out its accuse objectively and credibly.
- Screened for conflicts of interest. All conditional commission members are screened in writing and in a confidential group give-and-take most possible conflicts of interest. For this purpose, a "conflict of interest" ways any fiscal or other interest which conflicts with the service of the individual considering it could significantly impair the individual's objectivity or could create an unfair competitive advantage for any person or organization. The term "conflict of interest" means something more than than individual bias. There must be an interest, ordinarily financial, that could be directly affected by the work of the commission. Except for those rare situations in which the Academies determine that a conflict of involvement is unavoidable and promptly and publicly disclose the conflict of interest, no private can be appointed to serve (or continue to serve) on a committee of the institution used in the development of reports if the individual has a disharmonize of involvement that is relevant to the functions to be performed.
- Point of View is different from Conflict of Involvement. A point of view or bias is not necessarily a conflict of involvement. Committee members are expected to take points of view, and the Academies attempt to balance these points of view in a way deemed appropriate for the job. Committee members are asked to consider respectfully the viewpoints of other members, to reflect their own views rather than exist a representative of any organization, and to base of operations their scientific findings and conclusions on the evidence. Each committee fellow member has the correct to issue a dissenting opinion to the written report if he or she disagrees with the consensus of the other members.
- Other considerations. Membership in the NAS, NAE, or NAM and previous interest in Academies studies are taken into account in committee selection. The inclusion of women, minorities, and immature professionals are additional considerations.
Specific steps in the committee selection and approval process are as follows:
- Staff solicit an extensive number of suggestions for potential committee members from a wide range of sources, then recommend a slate of nominees.
- Nominees are reviewed and approved at several levels within the Academies; a provisional slate is so approved by the president of the National Academy of Sciences, who is also the chair of the National Research Council.
- The provisional commission list is posted for public comment in the Current Projects Organization on the Web.
- The conditional committee members complete background data and conflict-of-interest disclosure forms.
- The commission residue and conflict-of-involvement word is held at the showtime committee meeting.
- Whatsoever conflicts of interest or issues of committee balance and expertise are investigated; changes to the commission are proposed and finalized.
- Committee is formally canonical.
- Commission members continue to be screened for disharmonize of interest throughout the life of the commission.

Relevant features of Squeamish
I. Commission Membership
In terms of participation in committees, Prissy as well differs from other panels in that it includes lay members and public at-large. Lay members are defined as those with personal experience of using health or intendance services, or from a community affected by an established or soon to be considered guideline. In developing the guidelines, the Commission is the contained advisory group that considers the prove and develops the recommendations, taking into account the views of stakeholders. It may be a standing Committee working on many guideline topics, or a topic-specific Commission put together to work on a specific guideline. Dainty also advocates flexibility in calling for participation in the Committee. If needed for a topic, the Committee tin can co-opt members with specific expertise to contribute to developing some of the recommendations. For case, members with experience of integrating delivery of services beyond service areas may also exist recruited, peculiarly where the development of a guideline requires more than flexibility than "conventional organisational boundaries" permit. If the guideline contains recommendations about services, Squeamish could phone call upon individuals with a commissioning or provider background in addition to members from practitioner networks or local authorities.
Two. Bear witness
The NICE approach towards evaluating clinical testify differs from other approaches. In addition to clinical bear witness, the commission is implored to also have into account other factors, such as the demand to prevent discrimination and to promote disinterestedness. Similarly, NICE recognizes that non all clinical research could or should event in implementation; therefore, NICE has added an indication as to whether a procedure should only be tested in further enquiry or that information technology exist put frontward for implementation. Factors that might prevent research from beingness implemented in practice would be prove that the commission considers to be insufficient at the current time. A 'inquiry only' recommendation is made if the evidence shows that there are important uncertainties which may exist resolved with additional show (presumably from clinical trials or real earth settings).Testify may also indicates the intervention is dangerous and/or not efficacious, and the committee will make a recommendation, under those conditions, not to utilise the procedure.
III. Economic Evidence
An of import feature in the Nice framework is its use of economic show in guidelines development. There are ii main considerations in drawing conclusions from economic studies for a given intervention. The offset is that the methodology is sufficiently strong to avoid the possibility of double-counting costs or benefits. NICE recommends that the mode consequences are implicitly weighted should be recorded openly, transparently and as accurately equally possible. Cost–consequences analysis and so requires the decision-maker to decide which interventions represent the best value using a systematic and transparent process. A related process is that an incremental toll-effectiveness ratio (ICER) threshold be used whenever possible and that interventions with an estimated negative net present value (NPV) should not be recommended unless social values outweigh costs.
The 2nd consideration NICE put frontwards on using economic show in translating research to clinical practice/policy concerns cost-minimization procedures. The commission took care to avoid blindly choosing interventions with the lowest costs by declaring that toll minimization can be used only when the difference in benefits between an intervention and its comparator is known to be small and the cost difference is large. Given the criteria, Nice believes that toll-minimisation analysis is only applicable in a relatively small number of cases.
In sum, economic evidence estimating the value of the intervention should be considered aslope clinical evidence, but judgment past social values (policy) should likewise exist taken into account to avoid choosing intervention merely because it is offered at the lowest cost.
IV. Producing Guidelines from Evidence
The final stride in translating research evidence into practice and policy guidelines is drafting recommendations. Because many people read simply the recommendations, the wording must be concise, unambiguous and like shooting fish in a barrel to interpret into exercise by the intended audience. Equally a general rule, the committee recommends that each recommendation or bullet point within a recommendation should comprise only one primary action and be accessible as much as possible to a wide audience.
An important guideline explicitly stated by NICE is to indicate levels of doubtfulness in the bear witness. It is the only institution to accept created a "Research recommendations process and methods guide," which details the approach to be used to identify cardinal uncertainties and associated research recommendations. In considering which research intervention or evidence to put forward for recommendation, the commission established guidelines that includes iii levels of certainty:
1. Recommendations for activities or interventions that should (or should non) be used
2. Recommendations for activities or interventions that could be used
three. Recommendations for activities or interventions that must (or must not) be used.
Bibliography
- CDC (2012). Reducing Scientific Bias from Skilful Opinion in Guidelines and Recommendations Evolution.
- Grade Working Group (2015). From Evidence to Recommendations: Transparent and Sensible. Retrieved from: http://www.gradeworkinggroup.org/.
- Guyatt, One thousand.H. (2008). Course: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336.
- Ministry of Health of British Columbia (2014). Guidelines and Protocols Advisory Committee Handbook: Developing Clinical Practice Guidelines and Protocols for British Columbia.
- National Academies of Sciences, Technology & Medicine (2015). Our Study Process: Ensuring Independent, Objective Communication. Washington, D.C. Retrieved from: http://www.nationalacademies.org/studyprocess/.
- Quaseem, A. Towards International Standards for Clinical Do Guidelines. Presentation delivered past the Chair of the Guidelines for International Network, Scottish Charity.
- UK National Institute for Health and Care Excellence (2015). The Manual on Developing Nice Guidelines.
- Institute of Education Sciences (2015). What Works Clearinghouse. Retrieved from: http://ies.ed.gov/ncee/wwc/Publications_Reviews.aspx?f=All%20Publication%20and%20Product%20Types,3.
Source: https://www.ebpsociety.org/blog/education/204-development-guidelines-research-briefing-document